How Do Fuel Cells Work?

A generic fuel cell (ie typical battery) is any device that converts chemical energy into electrical energy. This utilizes two electrodes of dissimilar material and an electrolytic solution between the two. One material is oxidized with the free electrons traveling through the electrolyte and out through the electrode. Typically this electrode will be attached to an electrical circuit powered by the cell. Specifically, it could be linked to a motor that could drive an alternating current generator to provide a variable voltage and frequency electrical signal.
Polymer electrolyte membrane cells are somewhat different. These conduct hydrogen atoms. Specifically, single protons, not including the electrons, are what I am referring to. These cells are incredibly compact but susceptible to contamination/point defects. As little as ten ppm of CO contamination can significantly degrade the chemical process. Another problem is the temperature requirements of these cells. Some cells which use water cannot exceed 100C due to the water boiling off. This can be alleviated by using acids or Polybenzimidazole. As a result, a higher power density can be achieved. However, specific care must be exercised to ensure that these materials do not cause an unfavorable reaction with the cell elements.
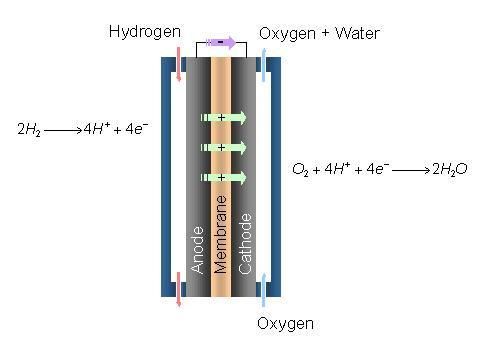
Another problem is the proper material for the gas diffusion layer of the cell. Currently, cell efficiency is highly limited by this material selection. A more electrically conductive material will increase cell efficiency; however, this material must be sufficiently hydrophobic to allow proper cell operation. Modern research is devoted to finding the best balance between these two goals.
Photo source: Stanford University





Member discussion