How do digital water clocks work?

Recently I started seeing Groupon offers and Amazon ads for "digital water clocks" that "run entirely off of the ions in the water."

Being skeptical, I did a little research. I found some pictures of the insides of these devices. Turns out, the answer should've been obvious. I assumed the clock would have an internal battery shorted into the circuit via water and that the apparatus was a scam. Instead, I discovered that it works according to the manufacturers' claims. These clocks use the ionic impurities in the water to provide power. However, it is incorrect to say that they're powered by water. Instead, they use something called "galvanic corrosion."
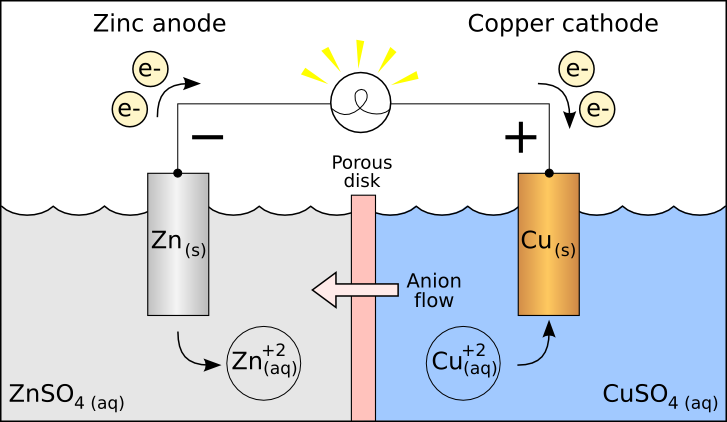
If you look at the above picture, you'll see two metal plates separated by a divider. That looks pretty similar to the chart on the left. What happens is that two electrodes are submerged in an electrolytic solution. In this case, tap water is used since it contains ionic impurities which allow for electrical conduction. These electrodes are composed of dissimilar metals. I don't know what each particular alarm clock contains, but we can pretend it's Zinc and Copper to match the chart.
Unfortunately, we're just using impure water, so our galvanic cell performance will be drastically reduced. I guess packing in a bottle of sulfuric acid detracts from the "green" image. Anyway, these metals possess different levels of electronegativity. That's a fancy word for saying that one metal wants more electrons than the other. As a result, electrons will flow from one electrode to the other and back through a porous membrane.
In doing so, electrons flow through the clock at a high enough volume to allow a simple IC chip to display to a 7-segment display. This gradually depletes one metal while adding progressively to the mass of the other metal. As a trivia point, galvanic corrosion is a significant problem in many industries, often requiring "sacrificial anodes." These are hunks of metal with a high susceptibility to galvanic corrosion. Instead of a pipe or wall corroding, these sacrificial anodes corrode. That brings me to my next point.
Over time, the electrodes would need replacing, which is probably not possible without destroying the clock. Oddly enough, using one of these clocks will cause the metals to leech out into the water that you must periodically pour down the sink. Zinc isn't especially bad for you in small quantities, but copper is toxic to plants and animals. Free copper ions can also combine with other things you might flush down the sink to make some much nastier compounds (like copper (II) chloride from the chlorination of free copper ions by free chlorine ions in an aqueous solution).
Regardless, the EPA sets a limit of 1.3mg of copper per liter of water for drinking water, so try to avoid it. Ultimately, you're paying a chunk of money for a clock with a built-in battery where you change the electrolyte by pouring it down the drain. It's an exciting novelty but not what I would call "green" and not "free energy."




Member discussion