Hafnium Discovery and Uses

Hafnium, Latin for “Copenhagen,” was discovered in Copenhagen, Denmark, in 1924. Dmitri Mendeleev first predicted its existence in 1869. Initially, there was significant controversy over who had found hafnium as the chemist Georges Urbain used a chemical technique with rare earth elements to produce a substance called celtium. However, x-ray spectrography revealed that the element did not contain 72 protons as expected. It was not until specific impurities in a zircon sample underwent the same spectrographic experiment that the element was observed. Hafnium was finally isolated by passing hafnium-tetra-iodine vapor over a heated tungsten filament. This purification process is still used today.
Hafnium’s primary use is in nuclear reactor control rods. It is used as a neutron absorber to control the rate of the nuclear fission reaction. Hafnium was selected for this role because it has a high microscopic cross-section of neutron absorption. Each hafnium atom has a high affinity for undergoing neutron absorption when in a neutron flux. Because of this, hafnium was selected as the material for most control rods used in the United States. Hafnium is formed into a blade that is attached to a control rod. Each control rod may have more than one blade. These control rods are then inserted into a nuclear fuel cell. The position of the control rod within the fuel cell will cause more or fewer neutron absorptions to occur within the cell. Since control rods are typically operated in groups, this will cause many fuel cells to have different neutron flux characteristics when control rods are utilized.
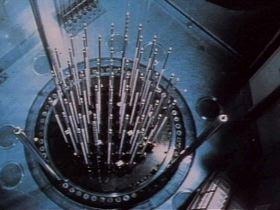
There are three primary uses for reactor control rods. One benefit is to provide a method for neutron flux shaping. By withdrawing control rods in groups and banks, the overall neutron flux profile of the reactor core can be altered to allow for specific design fuel burnout rates. By performing physics testing, different neutron fluxes can be calculated and engineered. This allows the operator to select various rod programming characteristics to account for core aging. As a result, areas of high fuel burnout will not develop due to an even flux profile. Overall, this allows for a longer core life.
Another use of control rods is for temperature control. In both pressurized water reactors and boiling water reactors, the height of the control rods is one of the major determining factors for temperature. By varying the rod height, the thermal non-leakage and fast non-leakage factors will change as more or fewer neutrons are absorbed. In doing this, the overall neutron flux of the core will change until it stabilizes at a new level. During this, the steam demand will have remained constant, so the thermal load on the reactor will not have changed. As a result, the steam demand will be less than the thermal output of the reactor. This will cause coolant temperatures to rise, increasing the overall plant temperature.
The final purpose of control rods is perhaps the most important: safety rods. In modern reactor design, some or all control rods will be designated safety rods. These rods will be driven into the core if a reactor plant casualty occurs. Since reactor power levels “at power” are primarily governed by steam demand, coolant circulation speed, or soluble boron levels in the coolant, there must be a quick way of rapidly shutting down the reactor. By inserting a large amount of negative reactivity in the form of neutron-absorbing control rods, reactor power levels will be instantly driven below the point at which heat is produced. Since heat will still be created from the decay of fission products, other systems must be used to reduce temperatures further.
Other uses for hafnium that may be involved in reactor construction and operation include insulation in Integrated Circuit chips that require dielectrics with a high “k” factor. This refers to the material’s specific capacitive ability to store energy in an electric field. With a higher k factor, the area can be reduced to allow circuit miniaturization while having the same overall capacitance. Hafnium is also used in plasma-cutting electrodes due to the material’s ability to shed electrons to form the plasma arc. Plasma cutting is the process by which metals of differing materials and thicknesses are joined together by a weld.

Hafnium is formed utilizing a process known as the Kroll Process. Hafnium tetrachloride impurities in zircon undergo a reduction reaction using magnesium or sodium at a temperature of about 1000C. One of the significant advantages of hafnium is that zircon is used in producing Zircalloy, which is used as nuclear fuel cladding inside reactors. Since it is contained in the byproducts of Zircalloy production, it was a simple matter for many manufacturers to refine hafnium on-site.
Hafnium is used in situations where cyclical stresses are regularly occurring. Hafnium is specifically used where thermal stresses separate oxide layers from their base metal. Chemically, hafnium is resistant to high alkali concentrations but is known to undergo reactions with oxygen, nitrogen, carbon, silicon, boron, and sulfur at higher temperatures. It is also possible to form tetrahalides when in the presence of halogens.
Hafnium has a high thermal resistance. Its melting point is approximately 2150C. Hafnium is often used in alloys for extremely high-temperature applications such as jet thrust nozzles or arc welders. Hafnium’s resistance to cyclical thermal stresses and high thermal conductivity make it ideal for rapid heat-up applications. Hafnium has a unique characteristic that makes it suitable for a neutron absorber. Each atom of hafnium-176 can undergo four neutron absorptions and still be a stable isotope. As a result, each hafnium control rod blade will last longer than the fuel it is inserted into.
Hafnium-diboride is a recently developed ceramic comprising hafnium and boron. It possesses an extremely high melting temperature of 3250C. It is also highly thermally conductive and has a high material strength. This may eventually become the material of choice for neutron absorption within reactor cores. It is also being investigated for use as ICBM heat shielding and leading edges of aircraft due to its ability not to undergo ablation. Ablation is when a material is removed due to vaporization, chipping, or erosion, typically when experiencing atmospheric reentry from space.





Member discussion